Transcriptomics & Epigenomics
“Epigenetics” is “the branch of biology which studies the causal interactions between genes and their products, which bring the phenotype into being” (Waddington, 1942).
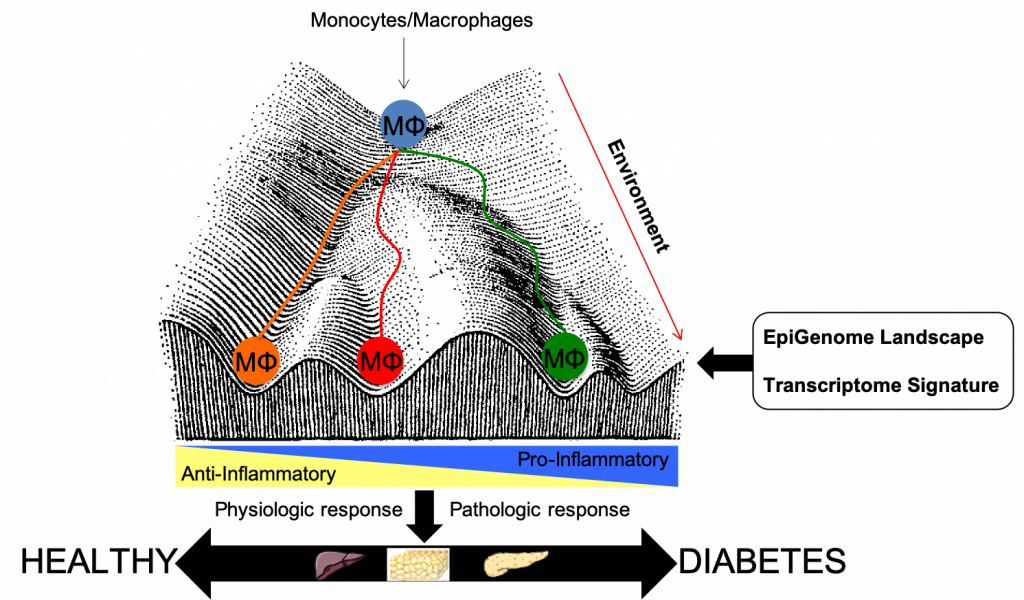
Monocytes and macrophages are key players in tissue homeostasis and immune responses. These tissue macrophages have trophic functions, maintaining tissue homeostasis and resolving inflammation. Monocytes provide a pool of macrophage precursors that is recruited upon inflammatory challenges to mediate host-defence against pathogens, foreign antigens, or tissue damage. Transcriptomic and Epigenetic processes tightly regulate cellular function in health and disease, such as obesity and type 2 diabetes. Recent studies from our team have allowed detailed characterisation of the transcriptional circuitry underlying monocyte and macrophage regulation (Toubal et al, JCI 2013, Dalmas et al. Nat Med 2016, Fan et al NatMed, Alzaid et al. JCI insight 2016, Drareni et al. Cell Reports 2018, Liang et al Nat Commun 2019). Upon differentiation and activation, genomic regions called enhancers are selected by lineage-determining and signal-dependent transcription factors. Enhancers are shown to be very dynamic, and activation of these enhancers underlies difference in gene transcription between monocytes, macrophages and their different subtypes. Epigenetic enzymes regulate the functioning of these cells and targeting epigenetic enzymes has been proven to be a valuable tool to dampen inflammatory responses.
Objectives
IMMEDIAB wants to establish a comprehensive understanding of the transcriptomic and epigenomic pathways that control monocyte and macrophage function and of the epigenetic enzymes involved in monocyte and macrophage differentiation and activation.
Future Directions
Our key challenges in the upcoming years will be to study epigenetic changes in human diabetes and to better understand how epigenetic pathways control the inflammatory repertoire in diabetic complications.
Publications
- Liang N, et al. Hepatocyte-specific loss of GPS2 in mice reduces non-alcoholic steatohepatitis via activation of PPARα. Nat Commun. 2019. doi: 10.1038/s41467-019-09524-z.
- Gilleron J et al. Rab4b Deficiency in T Cells Promotes Adipose Treg/Th17 Imbalance, Adipose Tissue Dysfunction, and Insulin Resistance. Cell Rep. 2018 doi: 10.1016/j.celrep.2018.11.083.
- Drareni K, et al. GPS2 Deficiency Triggers Maladaptive White Adipose Tissue Expansion in Obesity via HIF1A Activation. Cell Rep. 2018 doi: 10.1016/j.celrep.2018.08.032.
- Laurans L, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018 doi:10.1038/s41591-018-0060-4.
- Alzaid F at al. IRF5 governs liver macrophage activation that promotes hepatic fibrosis in mice and humans. JCI Insight. 2016 Dec 8;1(20):e88689. doi: 10.1172/jci.insight.88689.
- Fan R, et al. Loss of the co-repressor GPS2 sensitizes macrophage activation upon metabolic stress induced by obesity and type 2 diabetes. Nat Med. 2016. doi: 10.1038/nm.4114.
- Gaborit B, et al. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv208.
- Dalmas E at al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med. 2015 doi: 10.1038/nm.3829.
- Dalmas E, et al. . T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014. doi: 10.2337/db13-1511.
- Venteclef N et al. . Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015 doi: 10.1093/eurheartj/eht099.
- Toubal A, et al. SMRT-GPS2 corepressor pathway dysregulation coincides with obesity-linked adipocyte inflammation. J Clin Invest. 2013 doi: 10.1172/JCI64052.



