Interferon Regulatory Factor 5 represses oxidative respiration in human and murine macrophages by inhibition of mitochondrial matrix protein GHITM
Our latest paper on macrophage, mitochondria and IRF5 under metabolic stress is in preprint on Research Square ! 🤩
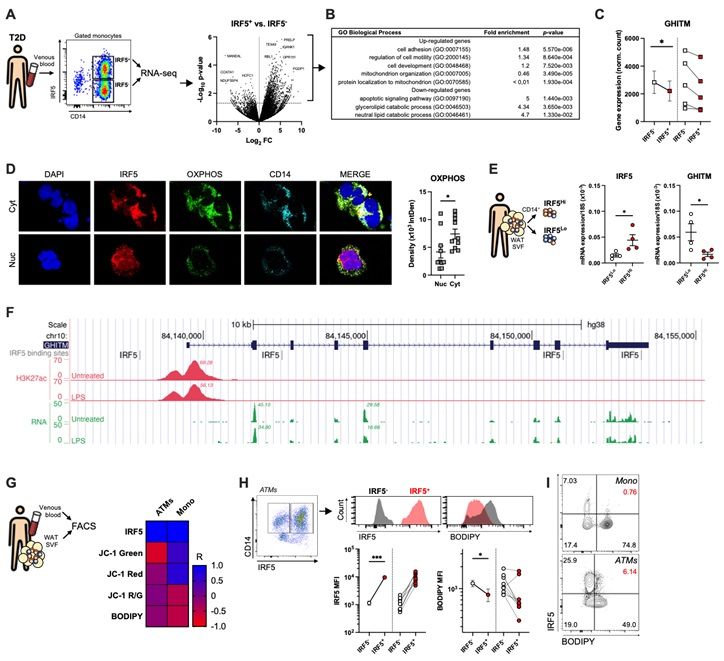
Over the course of diet-induced obesity, adipose tissue macrophage (ATM) populations transition from highly oxidative and protective to highly inflammatory and metabolically deleterious.
Here, we demonstrate that the Interferon Regulatory Factor (IRF)-5 is a key molecular switch mediating repression of macrophage oxidative capacity early in diet-induced obesity. ATMs from mice with a myeloid-specific deletion of IRF5 are characterised by increased mitochondrial activity and oxidative respiration compared to ATMs from wild-type mice. This hyper-oxidative phenotype is inducible and reversible in vitro by delivery of an inhibitory IRF5-decoy peptide and through IRF5 adenoviral overexpression, respectively.
In a data-driven approach, using public IRF5-cistrome data and in-house RNA-sequencing, we identified a transcriptional mechanism by which cellular oxidative capacity is repressed in an IRF5-dependent manner. The hyper-oxidative phenotype of IRF5-deficient macrophages is mediated by the Growth Hormone Inducible Transmembrane Protein (GHITM), known for maintaining mitochondrial architecture for optimal oxidative respiration.
Cas9-mediated simultaneous knock-down of GHITM and of IRF5 reverses the hyper-oxidative phenotype associated with IRF5-deficiency in vitro. IRF5-dependent regulation of GHITM expression and mitochondrial activity extends to human ATMs and monocytes from obese and diabetic patients.
While waiting for the official print, head to the following print to learn more about this work:
https://www.researchsquare.com/article/rs-477956/v1





